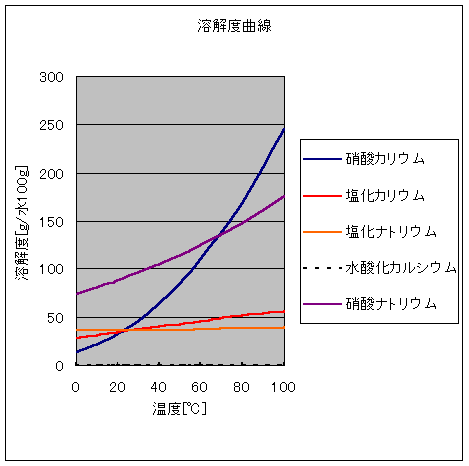
「固体の水に対する溶解度」水100gにとける無水物の(g)
§2 物質の状態
3 溶 液
(1)溶解と溶解度

「固体の水に対する溶解度」水100gにとける無水物の(g)
| ℃ | 0 | 10 | 20 | 25 | 30 | 40 | 50 | 60 | 80 | 100 |
| KNO3 | 13.3 | 22 | 31.6 | 37.9 | 45.6 | 63.9 | 85.2 | 109.2 | 168.8 | 244.8 |
| KCl | 28.1 | 31.2 | 34.2 | 35.9 | 37.2 | 40.1 | 42.9 | 45.8 | 51.3 | 56.3 |
| NaCl | 35.7 | 35.7 | 35.8 | 35.9 | 36.1 | 36.3 | 36.7 | 37.1 | 38 | 39.3 |
| NH4Cl | 29.4 | 33.2 | 37.2 | 39.3 | 41.4 | 45.8 | 50.4 | 55.3 | 65.6 | 77.3 |
| CuSO4 | 14 | 17 | 20.2 | 22.2 | 24.1 | 28.7 | 33.9 | 39.9 | 56 | 76.7 |
| (NH4)2SO4 | 70.5 | 72.6 | 75 | 76.4 | 77.8 | 80.8 | 84.5 | 87.4 | 94.9 | 101.7 |
| Ca(OH)2 | 0.189 | 0.182 | 0.175 | 0.17 | 0.16 | 0.141 | 0.128 | 0.122 | 0.106 | 0.1 |
| NaNO3 | 73 | 80.5 | 88 | 91.9 | 96.1 | 104.9 | 114.1 | 124.2 | 148.1 | 175.5 |